“The ubiquitin code written on the ubiquitin ligase HOIP regulates inflammation”
A small modifier protein ubiquitin regulates every aspect of biology. Our new study revealed a mechanism how inflammation is regulated by ubiquitination. The mechanism involves ubiquitin modification of the ubiquitin ligase HOIP, a known immune signaling regulator.
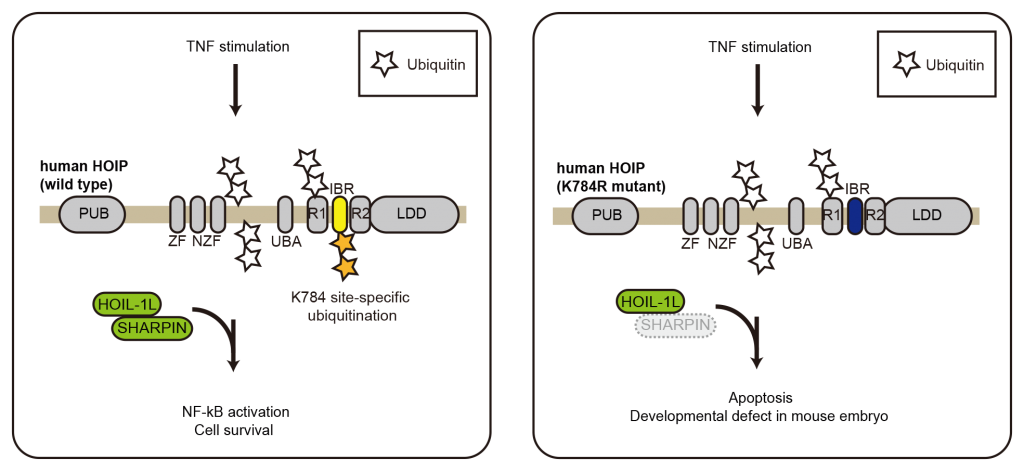
HOIP generates a special type of the ubiquitin code namely a linear (Met1-linked) ubiquitin chain and regulates inflammatory signaling cascades induced by various cytokines. An open question was how the ligase HOIP is regulated in upon cytokine stimuli in cells.
In this study, we tacked this question by focusing on the posttranslatioal modification of HOIP. We found that one ubiquitination site in HOIP is important in the regulation of TNF-induced apoptosis and NF-kB activation. By using a newly generated knockin mice in which the HOIP ubiquitination site is mutated, we found that HOIP ubiquitination at the site plays collaborative role with SHARPIN, one of the two partner proteins of HOIP.
In summary, we found that the modulation of HOIP by ubiquitination is one of the mechanisms how its downstream signaling cascade is regulated.
Title: Site-specific ubiquitination of the E3 ligase HOIP regulates apoptosis and immune signaling
Authors: Lilian M. Fennell, Carlos Gomez Diaz, Luiza Deszcz, Anoop Kavirayani, David Hoffmann, Kota Yanagitani, Alexander Schleiffer, Karl Mechtler, Astrid Hagelkruys, Josef Penninger, Fumiyo Ikeda
EMBO Journal
DOI: 10.15252/embj.2019103303
A link to the press release page (in Japanese):