Linear Ubiquitination in inflammation, cell death and autophagy
Keywords: Ubiquitin signal, E3 ligase, immune responses, cell death, autophagy
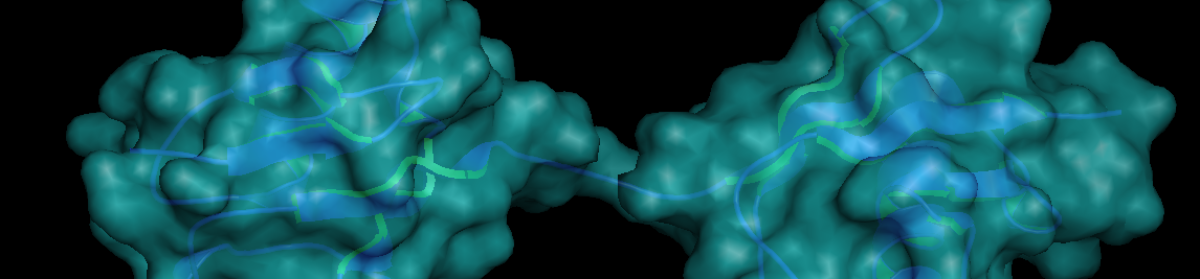
We are interested in ubiquitin – a dynamic modification that diversifies protein function by altering any of a number of features, including structure, stability and interacting partners. Given its broad range of effects and substrates, protein ubiquitination regulates and coordinates many biological processes at the molecular, cellular and organismal levels. Our group studies how ubiquitin networks control inflammation, cell death and autophagy. We use multiple approaches to tackle this question, from biochemistry techniques to genetically modified animal models. Ubiquitin molecules link together to form chains with different topologies on their target proteins, depending on which enzymes link them together. These different topologies recruit distinct complexes and effectors that specifically regulate various aspects of physiology. We are particularly interested in understanding the mechanisms and biology of a non-classical topology: linear ubiquitination (Figure 1).
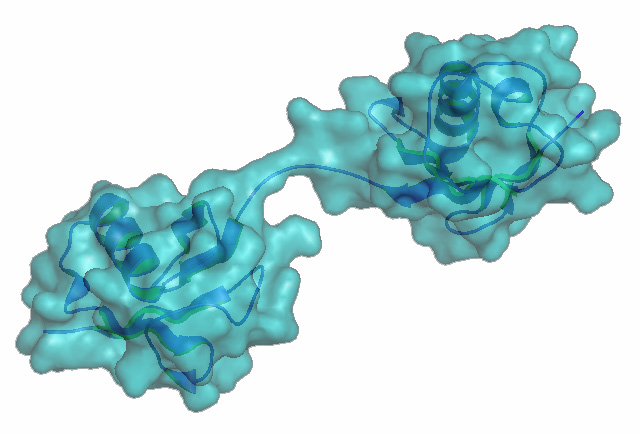
Figure 1. Met1-linked/ linear ubiquitin dimer.
The biological importance of LUBAC (Linear Ubiquitin Chain Assembly Complex)
LUBAC is thus far the only ligase known to generate linear ubiquitin chains. LUBAC consists of 3 proteins (HOIP, HOIL-1L and Sharpin), all of which are important for the adaptive immune signaling cascade, namely TNF-induced NF-kB activation, cell death and autophagy (Figure 2). Mutations in HOIP and HOIL-1 are associated with autoimmune disease as well as myopathy in humans. In mice, knockout of HOIP and HOIL-1L leads to embryonic lethality, whereas Sharpin-deficient mice suffer from systemic inflammation. In addition, we previously found that the homologous Linear Ubiquitin E3 Ligase (LUBEL) in flies regulates heat tolerance. We use mouse models to understand how these biological functions are regulated by the linear ubiquitination enzymes/complexes.
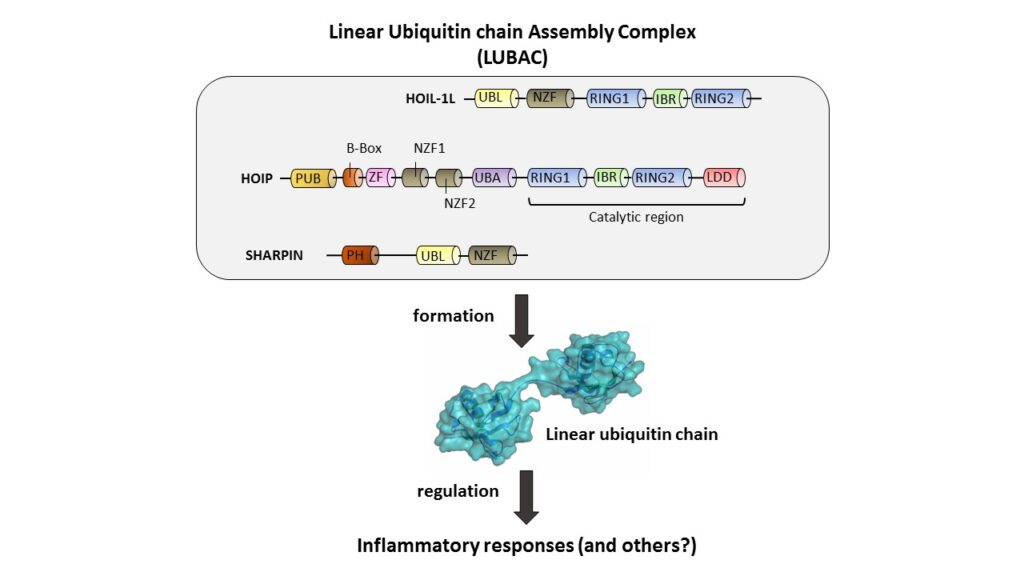
Figure 2. LUBAC reguating inflammatory reponses via linear ubiquitination.
Impact
Since essential enzymes regulating linear ubiquitination were found to be important for proper immunity and to control cancer development in humans, it is critical to understand how linear ubiquitination is regulated and how linear ubiquitination impacts diverse cellular responses. Our research is expected to contribute to the development of therapeutic strategies that correct or manipulate protein ubiquitination.
Projects
Mechanisms of linear ubiquitination by the LUBAC ligase complex
Linear ubiquitin chains are essential regulators of multiple immune signalling cascades. The LUBAC complex is the only known ubiquitin ligase complex capable of assembling these chains. However, it is unclear how LUBAC is regulated, activated, or recruited to signalling complexes. Furthermore, the mechanisms through which linear ubiquitin chains influence the activity of the substrates they are conjugated to are unknown. Our group is using a multidisciplinary approach combining biochemistry, cellular and chemical biology to unravel the mechanisms underlying linear ubiquitination. We are specifically interested in understanding how linear ubiquitin chains mediate the activation of enzymatic substrates, and the mechanisms in regulating the activity of LUBAC. Through these studies, we aim to unravel the mechanistic role of linear ubiquitin chains in immune signalling and develop experimental strategies that can be universally applied to study ubiquitination.
Linear ubiquitination in immune responses
In mammals, linear ubiquitination plays an important role in the immune responses and cell death. In human patients who suffer from autoimmune disease and myopathy were found to have mutations in genes responsible for the LUBAC components. In mouse models in which LUBAC component is deficient, apoptosis is abnormally increased. For example, apoptosis in multiple organs is increased in deficient mice of a LUBAC component Sharpin. By contrast, knockout of the LUBAC component, HOIP or HOIL-1L in mice leads to embryonic lethality accompanied by increased apoptosis in various tissues. While the involvement of LUBAC-dependent linear ubiquitination in the regulation of apoptosis and immune responses is clear, it remains to be understood how each LUBAC component regulates these events. Moreover, the roles of LUBAC in biology besides inflammatory responses and cell death remain largely elusive (Figure 1). We aim to understand the biological roles of linear ubiquitination using biochemical and cellular techniques coupled with genetically modified mouse models.
Ubiquitination and ubiquitin enzymes in autophagy
Autophagy is a pivotal lysosomal degradation pathway that protects cells from nutrient deprivation, as well as several other stress conditions (Figure 3). To ensure rapid activation, but at the same time to avoid degradation of essential material, it needs to be tightly controlled. One key mechanism to regulate autophagy is posttranslational modification of autophagy players with the small protein ubiquitin, ubiquitination. Even though ubiquitination is crucial for autophagy regulation, to date, only a handful of ubiquitin enzymes including LUBAC have been identified and characterized to regulate autophagy. In this project, we aim to characterize ubiquitin enzymes and their role in autophagy. In this way, we aim to broaden the current knowledge of how ubiquitination contributes to autophagy regulation.
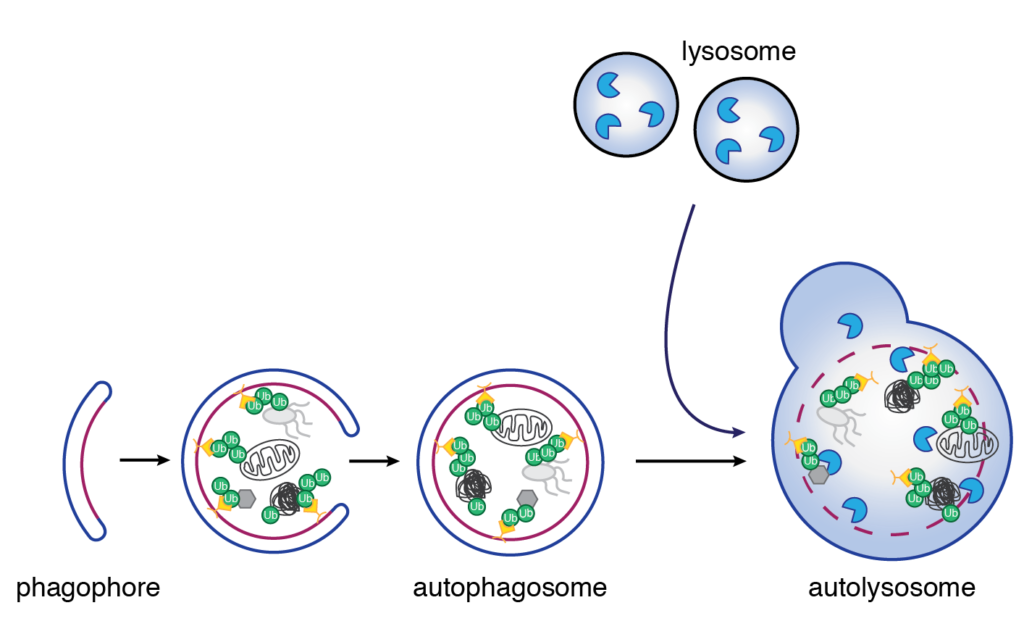
Figure 3.
Active grants
Fumiyo Ikeda
2021年度 武田報彰医学研究助成
2021-2025年度 科研費 基盤研究(A)
Kota Yanagitani
2022-2027年度 JST創発的研究支援事業
2022-2025年度 科研費 基盤研究(B)
Stephanie Roshni Kaypee
2024-2025年度 科研費 若手研究